The FDA Cosmetic Act: In-Depth Analysis of the Federal Food, Drug, and Cosmetic Act and Its Modern Impact on Cosmetic Regulation
- Evan Howard
- Apr 29, 2025
- 9 min read
The FDA Cosmetic Act, formally known as the Federal Food, Drug, and Cosmetic Act (FD&C Act), is the foundation of modern cosmetic regulation in the United States. Enacted in 1938, the FD&C Act was a direct response to mounting public health crises and the realization that earlier laws, like the Pure Food and Drug Act of 1906, were insufficient for protecting consumers from unsafe products. Over the decades, this law has been amended and expanded, most notably by the Modernization of Cosmetics Regulation Act of 2022 (MoCRA), to address the evolving landscape of the cosmetic industry and the increasing complexity of cosmetic products and their ingredients.

Why the FDA Cosmetic Act Was Enacted
The FD&C Act was born out of necessity. In the early 20th century, the United States faced a series of public health disasters linked to unregulated products. The most notorious was the 1937 Elixir Sulfanilamide tragedy, where a pharmaceutical company used diethylene glycol-a toxic solvent, resulting in over 100 deaths nationwide. This tragedy, along with other incidents involving unsafe cosmetics and drugs, swayed public opinion and forced Congress to act.
Prior to the FD&C Act, the Pure Food and Drug Act of 1906 provided only limited oversight, focusing mainly on food and drugs and lacking any real authority over cosmetics. As consumer products increased and became more complex, the need for a comprehensive regulatory framework became the necessity. The FD&C Act was designed to fill these gaps, providing the FDA with the authority to regulate not just food and drugs, but also cosmetics and medical devices, with the overarching goal of protecting public health and consumer safety.
Legislative Intent and Consumer Protection
The FD&C Act was crafted with several key objectives:
Preventing Adulteration and Misbranding: The law aimed to stop the distribution of products that were contaminated, unsafe, or labeled in a deceptive manner.
Requiring Scientific Proof of Safety: For the first time, manufacturers had to demonstrate the safety of new drugs before marketing them, setting a precedent for evidence-based regulation.
Extending Oversight to Cosmetics: By explicitly including cosmetics within its scope, the Act recognized the need for oversight of products applied to the human body for beautification, cleansing, or altering appearance.
Empowering the FDA: The Act gave the FDA the tools to inspect facilities, seize unsafe products, and take legal action against violators, fundamentally changing the landscape of consumer protection in America.
What the FDA Cosmetic Act Covers
The FD&C Act is a sweeping piece of legislation that governs a wide array of consumer products, including:
Foods and Food Additives
Dietary Supplements
Drugs (Prescription and Over-the-Counter)
Medical Devices
Tobacco Products
Cosmetics
Defining Cosmetics Under U.S. Law
Section 201(i) of the FD&C Act defines a cosmetic as:
“Articles intended to be rubbed, poured, sprinkled, or sprayed on, introduced into, or otherwise applied to the human body...for cleansing, beautifying, promoting attractiveness, or altering the appearance”.
This definition encompasses a wide range of products, such as:
Skin moisturizers, lotions, and creams
Perfumes and colognes
Lipsticks, foundations, and other makeup
Nail polishes and removers
Shampoos, conditioners, and hair treatments
Hair dyes and permanent wave products
Deodorants and antiperspirants
Cleansers and exfoliants
Importantly, the law distinguishes cosmetics from drugs, which are intended to diagnose, cure, mitigate, treat, or prevent disease, or to affect the structure or function of the body. Some products, such as anti-dandruff shampoos or acne treatments, may be classified as both cosmetics and drugs, depending on their intended use and claims.
Exclusions and Special Cases
The FD&C Act specifically excludes soap from the definition of cosmetics, provided it meets certain criteria related to composition and intended use. This distinction is important for manufacturers, as soaps are subject to different regulatory requirements.
Adulteration and Misbranding
Central to the FD&C Act are its prohibitions against the marketing of adulterated or misbranded cosmetics:
Adulteration: A cosmetic is considered adulterated if it contains harmful or poisonous substances, is prepared or held under unsanitary conditions, contains unauthorized color additives, or is otherwise unsafe for use.
Misbranding: A cosmetic is misbranded if its labeling is false or misleading, if required information is missing or not prominently displayed, or if the packaging fails to comply with established standards. The Act requires that all labeling be truthful, accurate, and not misleading in any way.
These provisions empower the FDA to act swiftly against products that pose a risk to consumers, whether due to contamination, unsafe ingredients, or deceptive marketing practices.
FDA Authority and Enforcement Powers
The FD&C Act grants the FDA broad authority to enforce its provisions, including:
Facility Inspections: The FDA can inspect manufacturing, processing, packing, and storage facilities to ensure compliance with safety and labeling requirements.
Warning Letters and Recalls: When violations are identified, the FDA may issue warning letters, request voluntary recalls, or, under MoCRA, initiate mandatory recalls if necessary.
Seizure and Injunction: The FDA can seize adulterated or misbranded products and seek court injunctions to prevent further distribution.
Civil and Criminal Penalties: Violators may face civil fines or criminal prosecution, especially in cases of willful or repeated non-compliance.
Labeling and Claims Requirements
Cosmetic labeling is tightly regulated to ensure consumers receive accurate information. Key requirements include:
Identity of the Product: The principal display panel must clearly state what the product is.
Name and Place of Business: The label must include the name and address of the manufacturer, packer, or distributor.
Net Quantity of Contents: The amount of product in the package must be declared in both metric and U.S. customary units.
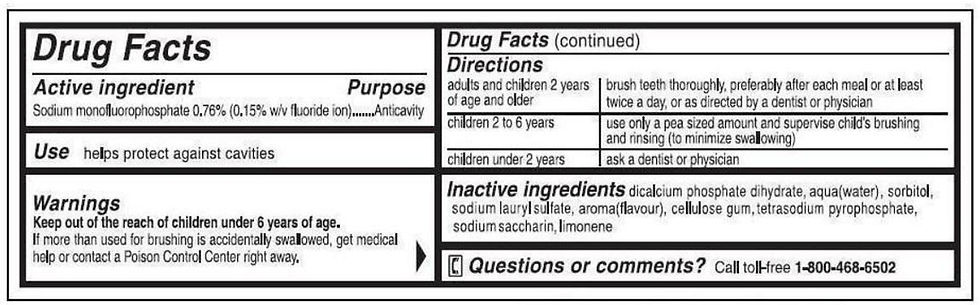
Ingredient Declaration: All ingredients must be listed in descending order of predominance, with specific rules for color additives, fragrances, and flavors.
Warning Statements: If a product could be hazardous if misused, appropriate warnings must appear on the label (e.g., for eye-area cosmetics or aerosol products).
Any therapeutic claims, such as treating skin conditions or altering bodily functions, may result in the product being regulated as a drug, subjecting it to stricter FDA requirements.
Pre-market and Post-market Oversight
Unlike drugs, cosmetics do not require FDA approval before being sold in the U.S. However, the FDA has robust post-market authority to monitor products, conduct facility inspections, and mandate recalls if products are found to be unsafe or non-compliant. Manufacturers are responsible for ensuring the safety of their products and for maintaining records that substantiate safety claims.
Modernization of Cosmetics Regulation: MoCRA and Its Impact
The Modernization of Cosmetics Regulation Act of 2022 (MoCRA) represents the most significant expansion of FDA authority over cosmetics since the original FD&C Act. MoCRA was enacted as part of the Consolidated Appropriations Act, 2023, and introduces sweeping changes to how cosmetics are regulated in the United States.
Key Provisions of MoCRA
Mandatory Facility Registration: All facilities that manufacture or process cosmetic products must register with the FDA, update their registration within 60 days of any changes, and renew registration every two years. This requirement enhances FDA oversight and traceability within the supply chain.
Product Listing: Responsible persons (manufacturers, packers, or distributors whose names appear on the product label) must list each marketed cosmetic product with the FDA, including detailed ingredient information. Updates must be provided annually, ensuring the FDA has current data on products available to consumers.
Adverse Event Reporting: MoCRA requires manufacturers to report serious adverse events associated with their cosmetic products, such as hospitalizations or significant injuries. This system enables the FDA to identify potential safety issues more rapidly and take action to protect public health.
Safety Substantiation: Companies must maintain records demonstrating that their products and ingredients are safe for use as directed. This requirement formalizes the industry’s responsibility for ensuring product safety.
Good Manufacturing Practices (GMP): MoCRA mandates that the FDA establish regulations for GMP specific to cosmetics, setting clear standards for facility cleanliness, quality control, and record-keeping.
Fragrance Allergen Labeling: The FDA is now required to develop regulations for labeling fragrance allergens in cosmetics, giving consumers more transparency about potential irritants or allergens in their products.
Mandatory Recall Authority: For the first time, the FDA has the power to mandate recalls of cosmetic products that pose a risk to consumer health, closing a critical gap in previous enforcement capabilities.
Facility Registration and Product Listing in Detail
MoCRA’s requirements for facility registration and product listing are detailed and comprehensive. Facilities must provide information such as:
Facility registration number
Parent company name (if applicable)
Facility DUNS Number
Contact information for responsible individuals
Product listings must include:
Facility registration number(s)
Name and contact number of the responsible person
Product name as it appears on the label
Cosmetic category/categories
Complete list of ingredients, including fragrances, flavors, and color additives
Product listing number (if previously assigned)
Type of submission (initial, update, renewal)
Optional information, such as images of product labels, product webpage links, and Unique Ingredient Identifiers (UNIIs), may also be submitted to enhance transparency and facilitate FDA oversight.
FDA Inspection and Investigation
The FDA regularly inspects cosmetic manufacturing and processing facilities to ensure compliance with the FD&C Act and MoCRA. Inspections focus on verifying adherence to GMP, accuracy of labeling, and the safety of ingredients and finished products. Facilities are expected to maintain detailed records and to provide them upon request during inspections.
Warning Letters, Recalls, and Penalties
When the FDA identifies violations, it may issue warning letters outlining the specific issues and required corrective actions. If companies fail to comply, the FDA can take further action, including:
Initiating voluntary or mandatory recalls of unsafe products
Seizing products found to be adulterated or misbranded
Seeking court injunctions to halt the distribution of non-compliant products
Imposing civil monetary penalties or pursuing criminal charges in cases of egregious or willful violations
Labeling Compliance and Guidance
The FDA provides detailed guidance for cosmetic labeling, covering aspects such as:
Prominence and conspicuousness of required information
Language and type size requirements
Ingredient declaration order and identification
Special rules for color additives, fragrance, and flavor ingredients
Requirements for multiunit or multicomponent packaging
Manufacturers must ensure that all labeling is truthful, not misleading, and provides consumers with the information necessary for safe and informed use of the product.
Impact of the FDA Cosmetic Act and MoCRA on the Cosmetic Industry
The FD&C Act, as amended by MoCRA, has significantly shaped industry practices. Cosmetic manufacturers and marketers must:
Register their facilities and products with the FDA
Maintain robust safety substantiation and adverse event reporting systems
Implement and adhere to Good Manufacturing Practices
Ensure transparent and accurate labeling, including the disclosure of potential allergens and complete ingredient lists
Respond promptly to FDA inquiries and enforcement actions
Consumer Confidence and Safety
By establishing clear regulatory standards and empowering the FDA with enhanced oversight and enforcement tools, the FD&C Act and MoCRA have bolstered consumer confidence in the safety and integrity of cosmetics sold in the United States. Consumers benefit from increased transparency, more rigorous safety standards, and improved mechanisms for addressing adverse events and product recalls.
Ongoing Challenges and Future Directions
Despite these advancements, the cosmetic industry continues to face challenges, such as:
Rapid innovation in ingredients and product formulations
The need for harmonization with international regulatory standards (e.g., EU regulations)
Addressing emerging concerns about potentially hazardous substances, such as PFAS, microbeads, and asbestos contamination in talc-based products
The FDA remains committed to updating its regulations and guidance to keep pace with scientific advancements and evolving consumer expectations.
Comprehensive Regulatory Framework
The FD&C Act, together with MoCRA and related legislation like the Fair Packaging and Labeling Act and the Microbead-Free Waters Act, provides a robust and adaptable framework for cosmetic regulation. Key features include:
Broad Definitions: The law’s definitions of cosmetics and drugs are intentionally broad, allowing for flexibility as new products and technologies emerge.
Clear Distinctions: The Act distinguishes between cosmetics, drugs, and other product categories, ensuring that each is regulated appropriately based on intended use and risk profile.
Flexible Enforcement: The FDA’s enforcement mechanisms are designed to be responsive, allowing the agency to address both acute safety threats and ongoing compliance issues.
Ongoing Modernization: With the passage of MoCRA, the FDA’s authority has been modernized to reflect current industry practices and consumer needs, including digital record-keeping, electronic submissions, and advanced ingredient tracking.
FDA’s Evolving Role and Industry Responsibilities
The FDA’s role has evolved from a reactive enforcer to a proactive regulator, emphasizing prevention, transparency, and rapid response to emerging risks. Manufacturers are now more accountable than ever, with explicit obligations for safety substantiation, adverse event reporting, and facility registration.
Consumers, too, play a role by reporting adverse events and staying informed about product recalls and safety alerts. The FDA provides resources and guidance to help both industry and consumers navigate the complex regulatory landscape.
The FDA Cosmetic Act, through the Federal Food, Drug, and Cosmetic Act and its amendments, stands as the bedrock of cosmetic regulation in the United States. It has evolved from a reactive response to public health crises into a sophisticated, proactive system that balances innovation with consumer safety. The recent implementation of MoCRA marks a new era of transparency, accountability, and rigorous oversight, ensuring that cosmetic products are safer and more transparent than ever before.
For manufacturers, compliance with the FDA Cosmetic Act is essential not only for legal operation but also for building consumer trust and maintaining a competitive edge in a dynamic, global marketplace. For consumers, the Act provides critical protections and assurance that the products they use daily are subject to rigorous oversight and safety standards.
As the cosmetic industry continues to innovate and expand, the FDA Cosmetic Act and its ongoing modernization will remain central to safeguarding public health and supporting informed consumer choice.
Howard Law is a business, regulatory and M&A law firm in the greater Charlotte, North Carolina area, with additional services in M&A advisory and business brokerage. Howard Law is a law firm based in the greater Charlotte, North Carolina area focused on business law, corporate law, regulatory law, mergers & acquisitions, M&A advisor and business brokerage. Handling all business matters from incorporation to acquisition as well as a comprehensive understanding in assisting through mergers and acquisition. The choice of a lawyer is an important decision and should not be based solely on advertisements. The information on this website is for general and informational purposes only and should not be interpreted to indicate a certain result will occur in your specific legal situation. Information on this website is not legal advice and does not create an attorney-client relationship. You should consult an attorney for advice regarding your individual situation. Contacting us does not create an attorney-client relationship. Please do not send any confidential information to us until such time as an attorney-client relationship has been established.



Comments