The Anticaries OTC Monograph: Foundation of Cavity Prevention in the U.S.
- Evan Howard
- May 13, 2025
- 8 min read
Dental caries-commonly known as tooth decay or cavities-remains one of the most prevalent chronic diseases globally. In the United States, the fight against cavities has been revolutionized by the widespread use of fluoride-containing products, especially toothpastes and mouth rinses. But behind every tube of fluoride toothpaste on the shelf is a rigorous regulatory framework: the FDA’s Anticaries OTC Monograph. This monograph not only ensures the safety and effectiveness of these products but also shapes the landscape of oral health for millions of Americans. The anticaries monograph defined, how it was developed, its current status, and its impact on public health and the oral care industry.
What Is the Anticaries OTC Monograph?
The anticaries OTC monograph is a comprehensive set of regulations issued by the U.S. Food and Drug Administration (FDA) that governs the marketing of over-the-counter (OTC) drug products designed to prevent dental caries. It specifies which active ingredients are permitted, their concentrations, acceptable dosage forms, required labeling, and quality standards. If a product meets all these conditions, it is considered “generally recognized as safe and effective” (GRASE) and can be sold directly to consumers without a New Drug Application (NDA).
The monograph system is unique to the U.S. and was established to streamline the approval and oversight of nonprescription drugs. For oral care, it means that products like fluoride toothpastes and mouth rinses can be widely available, affordable, and trusted by consumers-all while maintaining high standards for safety and efficacy.
The Origins and Development of the Anticaries Monograph
Early Days and Public Health Need
The roots of the anticaries monograph trace back to the 1970s, when the FDA launched a comprehensive review of all OTC drug products. The goal was to evaluate the safety and effectiveness of thousands of nonprescription medications, many of which had been on the market for decades without formal FDA review. Dental caries was-and remains-a major public health concern, so the need for effective, accessible preventive products was clear.
The OTC Drug Review Process
The development of the anticaries monograph followed the standard three-step OTC drug review process:
Advance Notice of Proposed Rulemaking (ANPR): The FDA published an ANPR in 1980, inviting public comment and gathering scientific data on ingredients used in anticaries products.
Tentative Final Monograph (TFM): In 1985, the FDA issued a TFM, proposing which ingredients and formulations would be considered GRASE and soliciting further input.
Final Monograph: After extensive review of comments, new data, and additional public input, the FDA published the final monograph in 1995, codified in 21 CFR Part 355.
This process spanned more than a decade and involved input from manufacturers, dental professionals, scientists, and consumer advocates. The result is a robust, science-based framework that continues to guide the formulation and marketing of anticaries products today.
What Does the Anticaries Monograph Cover?
Scope and Applicability
The anticaries monograph applies to all OTC drug products in forms suitable for topical administration to the teeth, such as toothpastes (dentifrices), gels, powders, and mouth rinses. It does not cover professional-use products or prescription-only formulations.
The monograph’s scope is broad, encompassing all products that claim to aid in the prevention of dental cavities. It sets clear boundaries: only products that comply with every aspect of the monograph are considered GRASE and not misbranded.
Approved Active Ingredients
A central feature of the anticaries monograph is its strict regulation of active ingredients. Only certain fluoride compounds, proven through decades of research to be safe and effective for cavity prevention, are permitted:
Sodium fluoride
Stannous fluoride
Sodium monofluorophosphate
Each ingredient is allowed only at specific concentrations, which vary depending on the dosage form (e.g., paste, gel, powder, rinse).
Detailed Ingredient Specifications
Sodium Fluoride
Toothpastes (Dentifrices): Must contain 850 to 1,150 ppm (parts per million) theoretical total fluorine in a gel or paste dosage form, corresponding to 0.188 to 0.254% sodium fluoride.
Powdered Dentifrices: Same fluoride range, but in a powdered form15.
Mouth Rinses: Typically contain 0.02% or 0.05% sodium fluoride, depending on whether they are intended for daily or weekly use1.
Stannous Fluoride
Toothpastes: Allowed at 0.4% stannous fluoride, providing a specific amount of available fluoride ion.
Sodium Monofluorophosphate
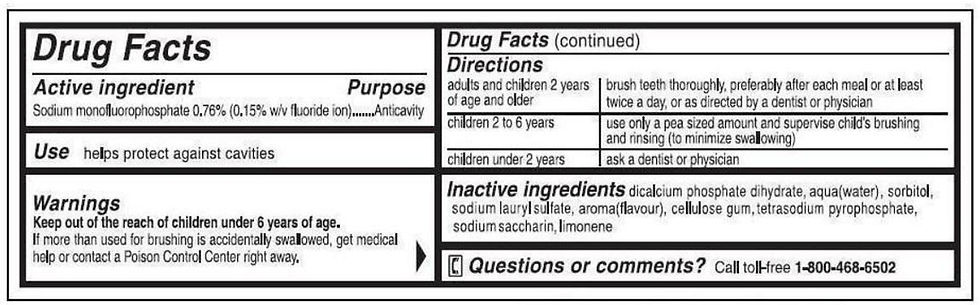
Toothpastes: Permitted at 0.76% sodium monofluorophosphate, yielding 1,000 ppm theoretical total fluorine.
These concentrations are carefully calibrated to maximize cavity prevention while minimizing the risk of adverse effects, such as dental fluorosis in children.
Dosage Forms and Packaging
The monograph allows a variety of dosage forms, including:
Toothpastes (Dentifrices)
Gels
Powders
Mouth Rinses
Effervescent Tablets and Treatment Rinse Powders (which must be diluted to the correct fluoride concentration before use)
Packaging requirements are also addressed to ensure product stability and accurate dosing. For example, certain fluoride rinses must be packaged in child-resistant containers to prevent accidental ingestion.
Labeling Requirements
One of the most important aspects of the anticaries monograph is its detailed labeling requirements. These rules are designed to inform consumers, promote safe use, and prevent misuse-especially in children.
Principal Display Panel
The front label must clearly identify the product as an anticaries drug and specify its intended use (e.g., “anticavity fluoride toothpaste”).
Indications
Permitted claims include:
“Aids in the prevention of dental cavities”
“Helps protect against cavities”
Warnings
Products must include specific warnings, such as:
“Keep out of reach of children under 6 years of age.”
For toothpastes: “If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.”
Directions for Use
Directions must be clear and age-appropriate, specifying the amount to use, frequency, and any special instructions for children.
Additional Labeling for Rinses
Fluoride rinses require extra warnings, such as:
“Do not swallow.”
“Supervise children as necessary until capable of using without supervision.”1
The monograph also details requirements for the Drug Facts panel, ingredient listing, and other mandatory information.
Testing and Quality Standards
To ensure product safety and efficacy, the anticaries monograph mandates specific testing procedures for fluoride dentifrices and other dosage forms. These include:
Assays for fluoride content: To verify that the product contains the correct amount of available fluoride ion throughout its shelf life.
Stability testing: To ensure the product remains effective and safe under normal storage conditions.
Microbiological testing: To confirm the absence of harmful microorganisms.
Manufacturers must maintain rigorous quality control and adhere to Good Manufacturing Practices (GMPs) as required by FDA regulations.
Professional Use and Combination Products
While the monograph is primarily intended for consumer (OTC) products, it also addresses labeling for professional-use products, such as fluoride treatments applied by dental professionals.
Combination products-such as those containing both a fluoride anticaries agent and a desensitizing ingredient like potassium nitrate-are permitted, provided each ingredient is included at an approved concentration and all labeling requirements are met.
How Are Ingredients Added or Changed?
The monograph system is designed to be both rigorous and adaptable. If a manufacturer or other stakeholder wishes to add a new active ingredient or change an existing condition, they must submit a petition with robust scientific evidence demonstrating the ingredient’s safety and efficacy for cavity prevention. The FDA then reviews the data, solicits public comment, and may amend the monograph through a formal rulemaking or administrative order process.
However, ingredients that have not been marketed in the U.S. or lack sufficient data cannot be added to the monograph. For example, some organic fluoride compounds used abroad are not included in the U.S. monograph because they lack a history of use and supporting data in this country.
The Rulemaking History: From Proposal to Final Monograph
The anticaries monograph’s journey from proposal to final rule is a case study in regulatory science:
1980: Advance Notice of Proposed Rulemaking (ANPR) published, gathering data and public input on anticaries ingredients.
1985: Tentative Final Monograph (TFM) issued, proposing which ingredients and formulations would be considered GRASE.
1995: Final Monograph published, codified in 21 CFR Part 355, establishing the definitive regulatory framework for OTC anticaries products.
Throughout this process, the FDA reviewed thousands of pages of scientific studies, public comments, and expert opinions. The result is a monograph that reflects the best available evidence and broad consensus among dental professionals, manufacturers, and public health experts.
Implementation and Enforcement
Once the final monograph was published, all OTC anticaries products had to comply with its requirements to remain on the market. Products that did not meet the monograph conditions were considered misbranded or unapproved new drugs and subject to enforcement action, including recalls or removal from the market.
Manufacturers are encouraged to comply as soon as possible, but all products must be in full compliance by the effective date specified in the monograph.
Impact on Public Health
The anticaries monograph has had a profound impact on oral health in the United States. By ensuring that only safe, effective, and properly labeled fluoride products are available to consumers, the monograph has:
Dramatically reduced the prevalence of dental caries: Widespread use of fluoride toothpaste is credited with significant declines in cavities among children and adults.
Increased access to preventive care: The monograph allows for affordable, effective products to be sold without a prescription, making cavity prevention accessible to all.
Promoted scientific innovation: The clear regulatory pathway encourages manufacturers to invest in research and development, knowing that products meeting monograph standards can reach the market efficiently.
The Monograph in Practice: What It Means for Consumers and Industry
For consumers, the anticaries monograph means that every tube of fluoride toothpaste or bottle of fluoride rinse on the shelf has met rigorous standards for safety, effectiveness, and labeling. There’s no need to decipher complicated ingredient lists or worry about unproven claims-products that comply with the monograph are backed by decades of scientific research and regulatory oversight.
For manufacturers, the monograph provides a clear, predictable pathway to market. As long as a product uses approved ingredients at specified concentrations, follows labeling requirements, and passes required tests, it can be sold without the time and expense of an NDA.
Ongoing Updates and Future Directions
The anticaries monograph is not static. The FDA continues to monitor new scientific evidence, safety data, and public health needs. Under the CARES Act, the process for updating monographs has become more efficient, allowing for faster responses to emerging issues or new research findings7.
For example, if new evidence emerges about a novel fluoride compound or a new dosage form, stakeholders can petition the FDA to amend the monograph. The agency will review the data, seek public input, and issue an administrative order if warranted.
International Perspective
While the U.S. monograph system is unique, many other countries have similar regulatory frameworks for oral care products. However, the specific ingredients, concentrations, and labeling requirements may differ. For example, some fluoride compounds permitted in Europe or Asia are not approved in the U.S. due to differences in regulatory standards and available data.
The Anticaries Monograph as a Model for Public Health
The FDA’s anticaries OTC monograph stands as a model of evidence-based regulation. By setting clear, science-driven standards for ingredients, dosages, labeling, and testing, it has made safe and effective cavity prevention accessible to all Americans. The monograph’s success is evident in the dramatic reduction of dental caries over the past several decades-a public health achievement that continues to benefit generations.
As science advances and new challenges emerge, the monograph system remains flexible and responsive, ensuring that oral care products keep pace with the latest research and public health needs. Whether you’re a consumer seeking the best protection for your family’s teeth or a manufacturer developing innovative oral care solutions, the anticaries monograph is your assurance of safety, efficacy, and trust.

Howard Law is a business, regulatory and M&A law firm in the greater Charlotte, North Carolina area, with additional services in M&A advisory and business brokerage. Howard Law is a law firm based in the greater Charlotte, North Carolina area focused on business law, corporate law, regulatory law, mergers & acquisitions, M&A advisor and business brokerage. Handling all business matters from incorporation to acquisition as well as a comprehensive understanding in assisting through mergers and acquisition. The choice of a lawyer is an important decision and should not be based solely on advertisements. The information on this website is for general and informational purposes only and should not be interpreted to indicate a certain result will occur in your specific legal situation. Information on this website is not legal advice and does not create an attorney-client relationship. You should consult an attorney for advice regarding your individual situation. Contacting us does not create an attorney-client relationship. Please do not send any confidential information to us until such time as an attorney-client relationship has been established.



Comments